- Systems
- Equipment
- Services
- Industries
- About Us
- Resources
- Contact Us
Figure 1: The OptiMix® wall-mounted baffle design
frees up room on and around the 200 gallon reactor head
permitting more ports for process analytical technology
.jpg?width=600&height=460&name=OptiMix%20Reactor%20(1).jpg)
Pharmaceutical companies are outsourcing the process development and synthesis of active pharmaceutical ingredients (APIs) for early clinical trials at an increasing rate. This presents a unique set of challenges for contract manufacturers who produce APIs. They must meet Food & Drug Administration (FDA) standards for current Good Manufacturing Practices (cGMP). They also must maximize reactor flexibility to serve dozens of different clients and produce scores of different drugs.
This means that contract manufacturers must invest in reactors that are not only easy to control and clean, but that also provide the broadest possible range of loading, mixing, and instrumentation options. "Because we are a contract manufacturer, we never know what we will be asked to produce, so we want our equipment as versatile as possible," explains Brian Swierenga, vice president of GMP Manufacturing at PharmaCore, Inc.
Operational flexibility was foremost in Swierenga's mind when he began planning PharmaCore's new cGMP pilot plant in High Point, N.C. The unit, which opened in early 2009, will house four glass-lined OptiMix® reactors from De Dietrich Process Systems, Inc.
The pilot plant is a major step for PharmaCore. Since it was founded in 2000, PharmaCore's chemists and engineers have specialized in manufacturing precommercial quantities of APIs. Although their customers discover and synthesize small-molecule APIs in their own laboratories, they are not commercial products. Most processes involve multiple steps and may have only marginal yields. PharmaCore assists with the production route and raises yields. The company focuses strictly on API synthesis rather than on formulating medicines with binders, excipients, and other chemicals.
Originally, PharmaCore produced gram-sized quantities suitable only for animal experiments, says Swierenga. A few years ago, it expanded to include cGMP laboratories capable of producing APIs in multikilogram quantities for early (Phase I) clinical trials on humans. The new pilot plant will be able to produce Phase II clinical trial quantities.
The existing production laboratory includes three validated cGMP cleanrooms. Each cleanroom can be configured for custom synthesis with large glass reactors and walk-in, hooded vacuum evaporators. PharmaCore analyzes reactions using tools such as high-performance liquid chromatography (HPLC), nuclear magnetic resonance (NMR), and liquid chromatography/mass spectrometry (LC/MS). All manufacturing takes place under cGMP guidelines for bulk pharmaceutical chemicals.
PharmaCore's existing laboratory is only large enough to produce kilogram quantities of APIs typically required for Phase I clinical trials. These studies usually involve trials on a group of 20 to 80 healthy volunteers to assess drug safety, tolerance, pharmacokinetics (drug breakdown in the body), and pharmacodynamics (drug effects on the body).
"Many of our customers want us to work with them on the next larger scale, and some potential new customers will only work with us if we can carry them through Phase II," says Swierenga. The company's new pilot plant will do that. "It allows us to move into Phase II clinical trial quantities and even small-scale Phase III testing. We couldn't do that before with our small reactor capacity." Phase II and Phase III clinical trials involve ever-increasing patient populations for evaluating drug efficacy as well as long-term safety. Pharmaceutical research companies generally consider Phase II to Phase III to represent a significant transition in the program, frequently sourcing Phase III supplies from large-scale vendors using commercial scale processes.
The new facility will include two 200 gallon (909 liter) reactors, one 300 gallon (1,364 liter) reactor, and a 500 gallon (2,273 liter) reactor. "We hope to recover 20 to 30 kilograms (44 to 66 pounds) of dry API after evaporation and purification from the 200 gallon reactors and 50 to 60 kilograms (110 to 120 pounds) from the 500 gallon reactor, which is a pretty decent size for clinical use," Swierenga says.
"We're putting in De Dietrich OptiMix reactors," he continues. "PharmaCore works with 30 to 40 different customers, and a typical production run lasts two to four weeks and includes three or four synthesis steps. We do almost any of the dozens of standard organic synthesis reactions, from alkylation to esterification to saponification."
Figure 2: The design frees up room around the reactor head for more ports,
thus expanding flexibility for multiple reactant additions
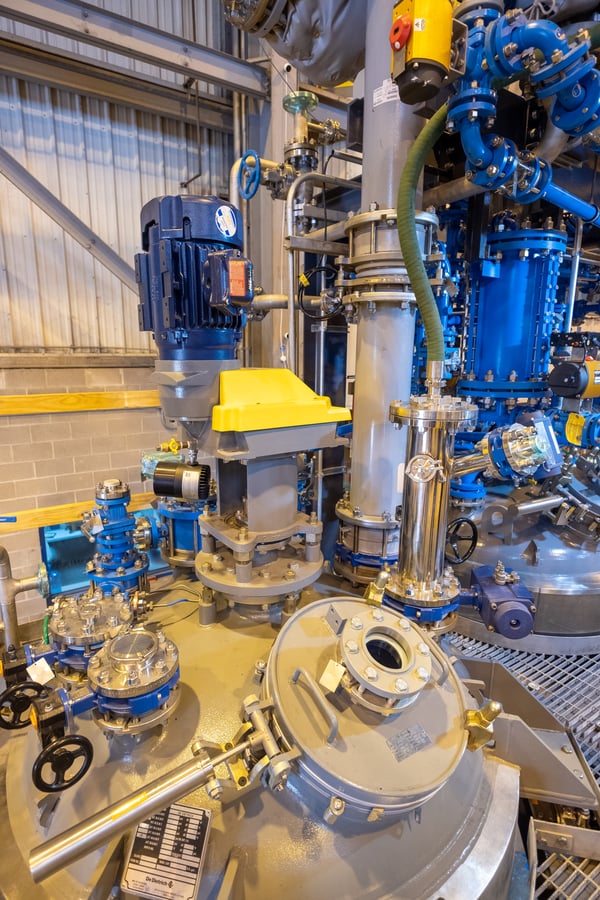
The patented design of PharmaCore's new, glass-lined OptiMix reactors incorporates three vertical baffles on the walls of the reactor shell. Side baffles are common in metal tanks. They prevent the formation of vortexes that spin the entire fluid mass but do very little actual mixing or shearing. In baffled tanks, on the other hand, the baffles create turbulence that enhances mixing. They also optimize heat transfer, suspension and distribution of solids and gases, and mass transfer.
Typical glass-lined reactors do not use straight flat baffles due to the challenge of attaching them to the reactor wall and encapsulating them with glass. Instead, they drop one or more baffles from the top head into the reactor. Under some conditions, these top-mounted baffles may not provide enough turbulence to suppress vortex formation. Vortices not only do a poor job of mixing, but they also splash liquid against reactor walls. In heated reactors, splashes soon turn into dried products that are difficult to remove from the reactor wall during cleanup.
De Dietrich developed the process to build wall-baffled, glass-lined reactors a few years ago. The company welds the baffles to the reactor wall prior to coating all steel surfaces with glass. The three baffles suppress vortexing and splashing. The symmetry of the three baffle/three mixing blade combination also minimizes bending loads that can deflect the agitator shaft and reduce seal life.
"The design really helps boost turbulence and improve top to bottom mixing," says Swierenga. "In relatively small reactors like the ones in our pilot plant, we would only be able to fit one or maybe two beaver tail baffles on the reactor head. Having three baffles in each reactor should really help with reactant turnover. I'm intrigued with some of the ways we'll be able to adjust the agitation for faster and more complete reactions."
Mounting baffles on the reactor shell also frees up room on and around the reactor head. "I've put in quite a few reactors, and it seems you never have enough nozzles on top," Swierenga says. "Each synthesis project may have its own requirement for probes and accessories, and you never know what you're going to need for future technology.
"The OptiMix design leaves more ports free for process analytical technology. The bigger pharmaceutical companies, and even the smaller ones, are beginning to follow reactions with infrared, raman, and other types of spectroscopy. You need decent size reactor ports to fit those probes, plus you still need room to take the usual temperature, pressure, and pH measurements," Swierenga concludes.
"The design also allows flexibility for multiple reactant additions," Swierenga continues, "Say if you need to do two or three reagent additions simultaneously. If we're charging a nonhazardous solid, for example, we can use the 14-by-18 inch (356-by-457 mm) porthole. If it is a hazardous solid, we can use one of the nozzles.
"If we're charging a liquid, we can either use a gravity feed, or pull a vacuum on the reactor and draw the liquid out of a tote or drum. We can add small amounts of catalysts or make controlled additions with metering pumps. Product exits from a valve at the bottom of the reactor cone, either through gravity or by pressurizing the reactor to push a slurry into a filter for separation," Swierenga states.
Swierenga also likes the cleanability of OptiMix reactors. "We do plenty of cleaning between batches, far more than a company making a handful of products, because we're a contract manufacturer and always moving from one product to the next," he explains. "We may clean every four to six days so we can prep the reactor for the next project."
The baffled design reduces the cleaning and validation time needed to comply with cGMP standards in several ways. First, as noted, the design reduces splashing so products do not bake onto heated reactor shells. Second, eliminating top head-mounted baffles eliminates difficult-to-clean top head pockets at the baffle/nozzle interface. Third, the nozzles and flanges have smooth, wide-radius surfaces for cleanability and workability. Fourth (and this is true for all glass-lined reactors), glass surfaces are smooth, simpler to clean, and more easily inspected for validation purposes. -Pharmaceutical Online The Magazine (Fall 2009 edition - www.pharmaceuticalonline.com)
Jacketed Reactors Offer Green Solution
Laboratory-scale jacketed reactors offer a cost-effective, "green" alternative to traditional manufacturing scale-up practices...read more

DE DIETRICH PROCESS SYSTEMS is the leading global provider of Process Equipment, Engineered Systems and Process Solutions for the fine chemical, chemical and pharmaceutical industry.
Quick Links